Chapter 5.2.2 / Why Are Some Gases Called Greenhouse Gases?
Why Are Some Gases Called Greenhouse Gases?
The purpose of this section is to explain which atmospheric gases are considered greenhouse gases and why.
Professional Development for Educators
Why Are Some Gases Called Greenhouse Gases?
What are greenhouse gases?
Earth’s atmosphere is composed of a variety of gases. Some are greenhouse gases (GHGs) and others are not.
GHGs in Earth’s atmosphere include:
- Water
- Carbon Dioxide
- Methane
- Nitrous Oxide
- Ozone
- CFCs/HCFCs
- Sulfur Hexafluoride
While many people know that carbon dioxide and methane are important GHGs, it is less common for individuals to know that water is the primary GHG in our atmosphere. It keeps the earth’s temperature relatively constant, especially compared to other planets that have extreme cold and extreme hot temperatures.
Other gases in Earth’s atmosphere that are NOT GHGs include:
- Nitrogen
- Oxygen
- Argon
- Helium
- Neon
- Hydrogen
- Carbon Monoxide
These non greenhouse gases, plus water, make up over 99% of the gases in the atmosphere. Only tiny amounts of helium, neon, hydrogen, and carbon monoxide gases actually exist in the atmosphere.
Why are these NOT greenhouse gases?
The BIG question here is “Why are nitrogen, oxygen, argon, helium, neon, hydrogen, and carbon monoxide NOT GHGs?” Most people don’t know that there is a simple answer, and that answer is related to the symmetry of the molecules. In the images below the line of symmetry is shown with a green line.
Why Symmetry?
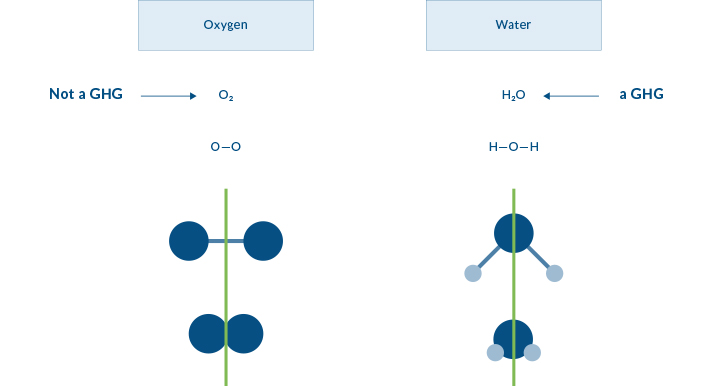
Atoms that make up the oxygen molecule (O2) include two oxygen atoms (O-O) joined together. In this illustration the ATOMS are represented by the balls, and the BOND joining the atoms are represented by sticks. Water (H2O) has two smaller atoms of hydrogen (shown as the lighter balls) joined to the oxygen atom in the middle by two bonds. Both oxygen and water form a symmetrical molecule, but one is a greenhouse gas and one is not.
Carbon Dioxide, CO2
Symmetrical or Unsymmetrical?

Carbon dioxide, whose structure is shown by these spheres, is also symmetrical.
So if these two GHGs are both symmetrical, how do they differ from non-GHGs? What about the symmetry is different?
The answer comes back to LIGHT!
Changes in the distance between atoms result when IR light is absorbed.
When longer lightwaves of infrared light (IR), shown as a yellow triangle in this illustration, hits a molecule’s bond, the bond stretches. A bond can be thought of as a spring between two balls. The IR light provides the energy that makes the bond stretch just as someone would have to pull on a spring to make it stretch. Stretching takes energy – from light.

CO2: Can its symmetry change?

So what happens to a carbon dioxide bond when IR light hits it? Just like other bonds, that bond stretches. If one bond stretches but not the other, the carbon dioxide molecule is no longer symmetrical. One bond is longer than the other so it loses its symmetry.
Only if symmetry changes can IR light be captured by the atmosphere

HERE IS THE BOTTOM LINE: A molecule is only a greenhouse gas if stretching can change its symmetry.
This happens because IR light can only change molecules that can absorb IR light energy. As it turns out, molecules whose symmetry does not change cannot absorb light in the IR region.
Does the symmetry of other gases change?
Hydrogen, Oxygen and Nitrogen are NOT greenhouse gases
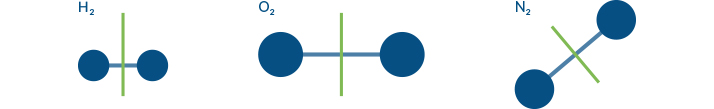
This illustration shows that the symmetry of these molecules cannot change. Even if the bonds were to stretch, they would not lose symmetry, so they cannot be GHGs.
These are NOT greenhouse gases
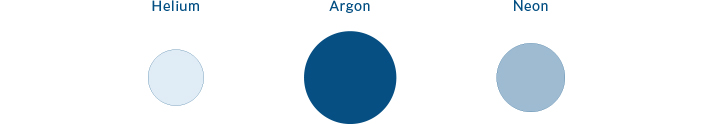 Of course molecules without bonds cannot change their symmetry at all, so none of these are GHGs.
Of course molecules without bonds cannot change their symmetry at all, so none of these are GHGs.
Can water's symmetry change?
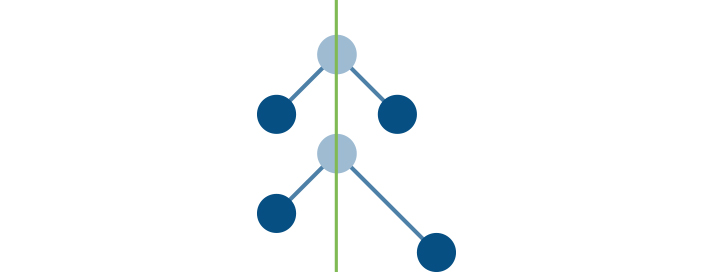
Water molecules can definitely change their symmetry so it is also an important GHG. However, there is naturally so much water in the atmosphere that the addition of more does not influence climate change quite like increases in carbon dioxide or other greenhouse gases.
Only molecules whose symmetry changes can be greenhouse gases
The key point to remember is this → only gases with molecules whose symmetry changes can be classified as greenhouse gases.
In the Classroom
Why Are Some Gases Called Greenhouse Gases?
Why Are Some Gases Called Greenhouse Gases? - 5.2.2 - PowerPoint PresentationMore Information and Resources
Why Are Some Gases Called Greenhouse Gases?
- Pacific Institute for Climate Solutions CO2 and the Greenhouse Effect (8:06 minutes) http://www.youtube.com/watch?v=sJ0eN_93l4k&feature=youtu.be
- Pacific Institute for Climate Solutions More than just CO2 (5:23 minutes) http://www.youtube.com/watch?v=fA9NwUiU4sU&feature=youtu.be
- Changing Climates, Colorado State University, Why Greenhouse Gases Make the Planet Warmer http://changingclimates.colostate.edu/movies/scott_denning_796kbits.mov
- An overview of GHGs: http://en.wikipedia.org/wiki/Greenhouse_gas
